

Imagine that this ion is placed next to a positive ion. Ceregrzyn M, Kamata T, Yajima T, Kuwahara A: Biphasic alterations in gastrointestinal transit following endotoxaemia in mice.\).Goncalves J, Wasif N, Esposito D, Coico JM, Schwartz B, Higgins PJ, Bockman RS, Staiano-Coico L: Gallium nitrate accelerates partial thickness wound repair and alters keratinocyte integrin expression to favor a motile phenotype.Mitsui T, Kondo T: Assessing nitrate metabolism in the intestinal tract by measuring breath nitric oxide and nitrous oxide, and its clinical significance.Susilo R, Korting HC, Strauss UP, Menke G, Schuster O, Menke A: Rate and extent of percutaneous absorption of sertaconazole nitrate after topical administration.Rachid MA, Camargos ER, Barcellos L, Marques CA, Chiari E, Huang H, Tanowitz HB, Teixeira MM, Machado CR: Blockade of endothelin ET(A)/ET(B) receptors favors a role for endothelin during acute Trypanosoma cruzi infection in rats.Tsikas D: Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids.Miyado T, Tanaka Y, Nagai H, Takeda S, Saito K, Fukushi K, Yoshida Y, Wakida S, Niki E: Simultaneous determination of nitrate and nitrite in biological fluids by capillary electrophoresis and preliminary study on their determination by microchip capillary electrophoresis.
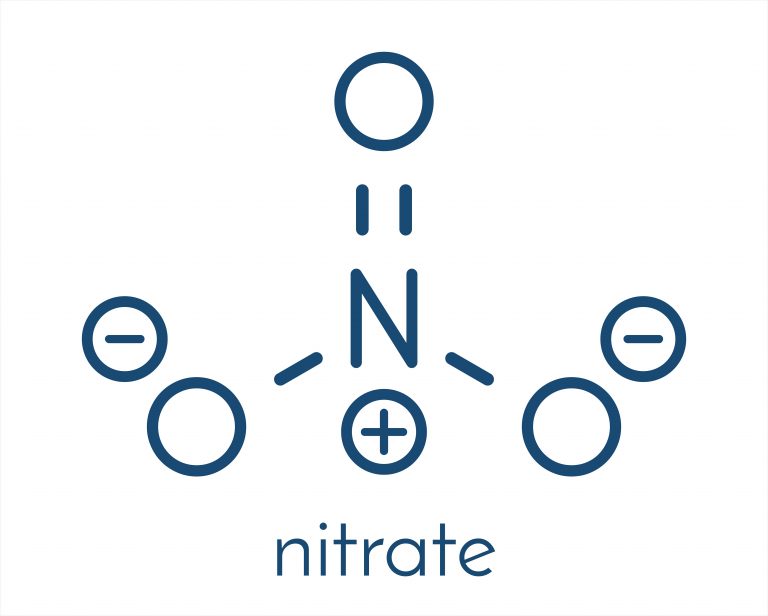

LC-MS/MS Spectrum - Nitrate Quattro_QQQ 10V, Negative-QTOF (Annotated) Predicted Kovats Retention Indices Underivatized Metabolite Predicted Collision Cross Sections Predictor Nitrate Exposure (MarkerDB: MDB00030005).Amyotrophic lateral sclerosis (MarkerDB: MDB00030005).Preeclampsia/Eclampsia (MarkerDB: MDB00030005).Methemoglobinemia can be treated with methylene blue. This condition is called methemoglobinemia and can lead to a lack of oxygen in tissues. Nitrites oxidize the iron atoms in hemoglobin from Ferrous Iron (2+) to Ferric Iron (3+), rendering it unable to carry oxygen. In particular, nitrate toxicosis in humans occurs through enterohepatic metabolism of nitrates to ammonia, with nitrite being an intermediate. Organic compounds containing the nitro functional group (which has the same formula and structure as the nitrate ion save that one of the O2 atoms is replaced by the R group) are known as nitro compounds. Nitrates should not be confused with nitrites, the salts of nitrous acid. The nitrate ion carries a negative one formal charge. The nitrate ion is a polyatomic anion with the empirical formula NO3- and a molecular mass of 62.01 daltons it consists of one central nitrogen atom surrounded by three identical oxygen atoms in a trigonal planar arrangement. In organic chemistry the esters of nitric acid and various alcohols are called nitrates. In inorganic chemistry, a nitrate is a salt of nitric acid.


 0 kommentar(er)
0 kommentar(er)
